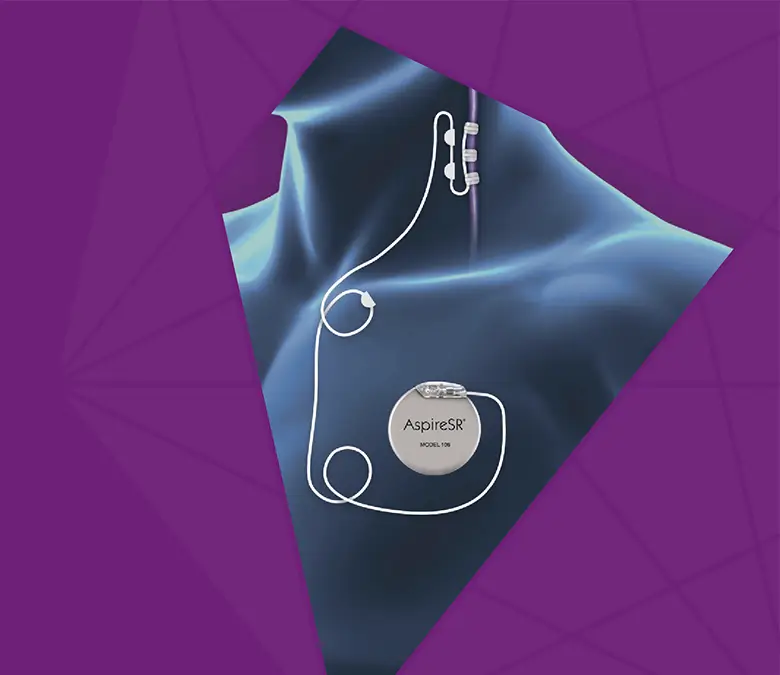
VNS Therapy™ has no drug interactions and does not cause drug-related toxic central nervous system side effects.
Common side effects include hoarseness or change in voice tone, shortness of breath, sore throat, and coughing. Most side effects associated with VNS Therapy occur only during stimulation, tend to diminish over time, or are eliminated by adjusting parameter settings.
The most common side effect of the surgical procedure is infection. Children under 12 may have a greater risk of infection than those 12 years of age and older and may be more likely to experience lead damage due to higher activity levels and the potential to manipulate the lead.
For more safety information click here.


.webp?width=100&height=98&ext=.webp)


