Connectors for CPB
A large variety of connectors, both straight and Y, with and without luer lock
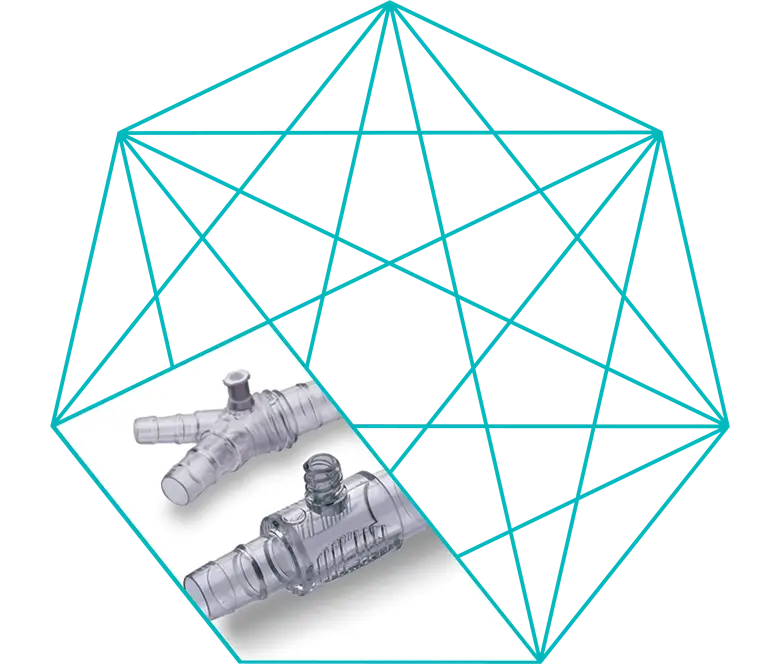
Connectors
Connectors are designed to allow connection of tubing and parts of the cardiopulmonary bypass (CPB) system. The devices are single use, non-toxic, apyrogenic, supplied sterile. The benefit of connectors is to ensure the continuity of the CPB circuit.
The following device models are available:
| Straight equal connectors | Straight reducing connectors | ||
|
1/2" x 1/2" |
1/2" x 1/2" w luer lock |
1/2" x 3/8" |
1/2" x 3/8" w luer lock |
|
3/8" x 3/8" |
3/8" x 3/8" w luer lock |
1/2" x 1/4" |
1/2" x 1/4" w luer lock |
|
1/4" x 1/4" |
1/4" x 1/4" w luer lock |
5/8" x 3/8" |
N/A |
|
3/16" x 3/16" |
3/16" x 3/16" w luer lock |
1/4" x 3/16" |
1/4" x 3/16" w luer lock |
|
1/4" x luer slip |
N/A |
3/8" x 1/4" |
3/8" x 1/4" w luer lock |
|
1/4" x luer lock |
N/A |
||
|
3/16" x luer slip |
N/A |
||
|
3/16" x luer lock |
N/A |
||
| Y equal connectors | Y reducing connectors | ||
|
1/2" x 1/2" x 1/2" |
1/2" x 1/2" x 1/2" w luer lock |
1/2" x 1/2" x 3/8" |
1/2" x 1/2" x 3/8" w luer lock |
|
3/8" x 3/8" |
3/8" x 3/8" x 3/8" w luer lock |
1/2" x 1/2" x 1/4" |
1/2" x 1/2" x 1/4" w luer lock |
|
1/4" x 1/4" x 1/4" |
1/4" x 1/4" x 1/4" w luer lock |
1/2" x 3/8" x 3/8" |
1/2" 3/8" x 3/8" w luer lock |
|
3/16" x 3/16" x 3/16" |
3/16" x 3/16" x 3/16" w luer lock |
1/2" x 3/8" x 1/4" |
1/2" x 3/8" x 1/4" w luer lock |
|
|
|
3/8" x 3/8" x 1/4" |
3/8" x 3/8" x 1/4" w luer lock |
|
|
|
3/8" x 1/4" x 1/4" |
3/8" x 1/4" x 1/4" w luer lock |
|
|
|
1/4" x 3/16" x 3/16" |
1/4" x 3/16" x 3/16" w luer lock |
| Parallel Y connectors | Adaptation Connector | ||
|
1/2" x 3/8" x 3/8" |
N/A |
3/8" to 1/4" adaptor |
N/A |
|
3/8" x 3/8" x 3/8" |
N/A |
|
|
|
3/8" x 1/4" x 1/4" |
N/A |
|
|
Safety Information
Important Safety Information for Cardioaccessories
1. INDICATIONS FOR USE / INTENDED PURPOSE
Suction Wands are intended to be used to remove fluids from the surgical field.
Connectors are intended to allow the connection between the CPB circuit components.
The Gas Filter is intended to provide filtration in the gas lines of the cardiopulmonary bypass (CPB) circuit.
The Vacuum Relief Check Valve is intended to be used during cardiopulmonary bypass surgery to establish one-way suction flow.
The VAVD valve is intended to control pressure during the use of hard-shell venous reservoir/cardiotomy reservoirs during CPB or post-operative chest drainage.
All of these devices are intended to be used for 6 hours or less.
2. CONTRAINDICATIONS
No contraindications are known if the device is used for the purpose described and in accordance with the stated operating conditions.
Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use. For a complete listing of warnings, please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
5. PERFORMANCE INFORMATION
The Suction Wands are devices designed to aspirate fluids from the surgical field.
The Connectors are devices designed to allow connection of tubing and parts of the cardiopolmunary bypass (CPB) system.
The Gas Filter is a device for the filtration of gases during surgical procedures.
The Vacuum Relief Check Valve is a combination valve designed to help relieve.excessive negative and positive pressure, and prevent retrograde flow in suction and vent lines.
VAVD VALVE is a relief valve that guards against negative and positive pressure.
The devices are single use, non-toxic, non-pyrogenic, supplied STERILE and individually packaged. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Not approved in all geographies. Please consult your labeling.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) ITALY

Essenz™ Perfusion System
Enter the New Era of Perfusion
Built on LivaNova’s 50-year legacy of safety and reliability, Essenz supports the Perfusionist in doing what is best for each patient, and allows the entire heart team to continuously improve their clinical practice.
Please Note: Essenz information available only in selected geographies.