Kids™ and Lilliput™ Pediatric Oxygenators
Small, sensitive neonatal and pediatric patients deserve great attention and dedicated perfusion solutions.
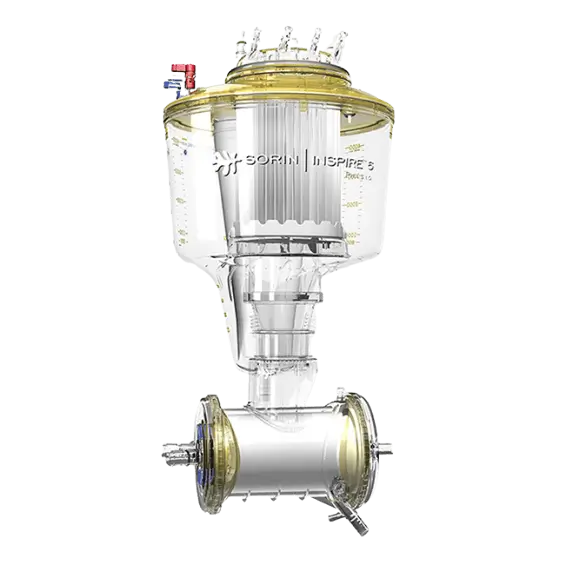
Kids™
Kids pediatric oxygenators are designed to minimize hemodilution and reduce foreign surface area in contact with blood with a patient tailored O2/CO2 transfer, KIDS provide adequate perfusion support to pediatric patients.1,2
-
For neonates, the D100 oxygenator is the world smallest neonatal oxygenator in the world. It has the smallest priming volume of any pediatric oxygenators in clinical use, just 31 ml.3,4
-
For infant patients, the D101 oxygenator and D131 arterial filter offer both high performance and low priming volume: only 115 mls for patients up to 2.5 LPM blood flow.5

| D100, D101, D130, D131 | |
| Advanced Reservoir |
The D100 and D101 reservoirs include features optimizing performance1,3,5
|
| Continuous Purge | The D130 and D131 arterial filters purge lines are designed to contemporaneously purge the filter on both sides to make priming fast and easy.2 |
| High Biocompatibility | LivaNova has designed a uniform biocompatible surface treatment that feature phosphorylcholine (PC).6 |
| Minimized Surface Area |
The D100 and D101 have a circumferential blood flow path design to maximize O2/CO2 exchange efficiency of the membrane bundle. As a result, foreign surface area exposure is minimized, without affecting the O2 and CO2 transfer.1,7
D100 small membrane surface area, in clinical use, allows precise CO2 control at lower rates, avoiding hyporcarbia and hyperoxia.2,7 |
References
1. Lawson et al. A clinical evaluation of the Dideco Kids D100 neonatal oxygenator. Perfusion 2008; 22: 39–42
2. Napoleone et al. Initial clinical experience with Dideco Kids D100 neonatal oxygenator. Journal of Cardiovascular Medicine 2008, 9:716–718
3. Bojan Recent Achievements and Future Developments in Neonatal Cardiopulmonary Bypass. Paediatr Anaesth. 2019 May;29(5):414-425. doi: 10.1111/pan.13597. Epub 2019 Mar 21.
4. Marupudi et al. In-Vitro Evaluation of Two Types of Neonatal Oxygenators in Handling Gaseous Microemboli and Maintaining Optimal Hemodynamic Stability During Cardiopulmonary Bypass. Braz J Cardiovasc Surg 2016;31(5):343-50
5. Mathis et al. Evaluation of four pediatric cardiopulmonary bypass circuits in terms of perfusion quality and capturing gaseous microemboli. Perfusion. 2012 Nov;27(6):470-9.
6. De Somer F, Van Belleghem Y, Caes F, Francois K, Van Overbeke H, Arnout J et al. Tissue factor as the main activator of the coagulation system during cardiopulmonary bypass. J Thorac Cardiovasc Surg 2002;123:951–8
7. Pouard et al. Pouard et al. Clinical evaluation of a new hollow fiber membrane oxygenator for neonatal cardiopulmonary bypass. ITBM-RBM Volume 27, Supplement 1, December 2006, Pages S19-S211. Lawson et al. A clinical evaluation of the Dideco Kids D100 neonatal oxygenator. Perfusion 2008; 22: 39–42
Safety Information
Summary of Safety & Performance Information for KIDS
1. INDICATIONS FOR USE / INTENDED PURPOSE
Models: D100 and D101
The devices are indicated for neonatal and Infant patients undergoing surgical procedures requiring cardiopulmonary bypass.
The devices are extracorporeal circulation devices used to replace the patient respiratory function during cardiopulmonary bypass procedures. They are used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the blood, and to cool or warm the blood during cardiopulmonary bypass procedures.
The hard-shell venous reservoirs are extracorporeal circulation devices allowing oxygenators to exploit gas exchange function of patient lungs during cardiopulmonary bypass procedures, providing dynamic patient’s venous blood collection. They also defoam and filter venous blood and suction blood through a filtering system made of antifoam agent and filter screen.
The devices must be to be used up to 6 hours or less.
2. CONTRAINDICATIONS
No contraindications are known if the device is used for the purpose described and in accordance with the stated operating conditions. Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use provided in the Instructions for Use. For a complete listing of warnings please refer to the Instructions for Use which accompany each product
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product
5. ADVERSE EVENTS
The following table summarizes harms potentially arising during the use of the medical device, including those related to the intrinsic risks of extracorporeal circulation: Hypoxia / Hypercarbia / Hypothermia / Hypoperfusion / Hypovolemia / Embolism / Sepsis / Fever / Systemic Inflammatory Response Syndrome (SIRS) / Allergic reactions / Hypersensitivity reactions / Irritation / Cytotoxic reactions / Genetic mutation / Cancer / Reprotoxic effects / Hemolysis / Impaired hemostasis / Infection (user or other persons) / Skin tears (user or other persons) / Bruise or Contusion (user) / Environmental contamination
6. PERFORMANCE INFORMATION
The devices are designed to come into contact with patient blood and are single use, non-toxic, non-pyrogenic, supplied sterile in individual packaging. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product. Not available in all geographies. Consult your labeling.
Legal Manufacturer:
Sorin group italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) Italy
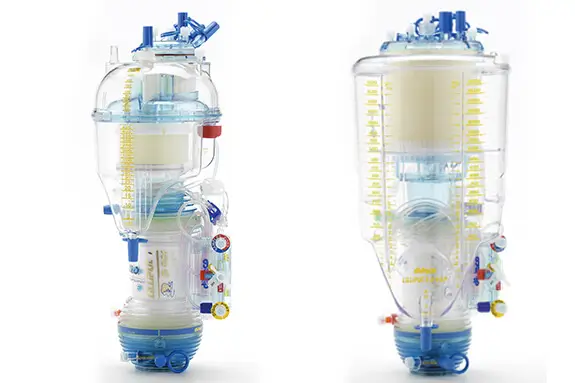
Lilliput™
Lilliput is a range of dedicated open system oxygenators with 3 decades of experience supporting pediatric patients during CPB.
Safety Information
Summary of Safety & Performance Information for LILLIPUT
1. INDICATIONS FOR USE / INTENDED PURPOSE
Models: LILLIPUT 1, LILLIPUT 2
The devices are indicated for neonatal and Infant patients undergoing surgical procedures requiring cardiopulmonary bypass
The devices are extracorporeal circulation devices used to replace the patient respiratory function during cardiopulmonary bypass procedures. They are used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the blood, and to cool or warm the blood during cardiopulmonary bypass procedures.
The hard-shell venous reservoirs are extracorporeal circulation devices allowing oxygenators to exploit gas exchange function of patient lungs during cardiopulmonary bypass procedures, providing dynamic patient’s venous blood collection. They also defoam and filter venous blood and suction blood through a filtering system made of antifoam agent and filter screen
The devices must be to be used up to 6 hours or less
2. CONTRAINDICATIONS
No contraindications are known if the device is used for the purpose described and in accordance with the stated operating conditions. Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use provided in the Instructions for Use. For a complete listing of warnings please refer to the Instructions for Use which accompany each product
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product
5. ADVERSE EVENTS
The following table summarizes harms potentially arising during the use of the medical device, including those related to the intrinsic risks of extracorporeal circulation.: Hypoxia / Hypercarbia / Hypothermia / Hypoperfusion / Hypovolemia / Embolism / Sepsis / Fever / Systemic Inflammatory Response Syndrome (SIRS) / Allergic reactions / Hypersensitivity reactions / Irritation / Cytotoxic reactions / Genetic mutation / Cancer / Reprotoxic effects / Hemolysis / Impaired hemostasis / Infection (user or other persons) / Skin tears (user or other persons) / Bruise or Contusion (user) / Environmental contamination
6. PERFORMANCE INFORMATION
The devices are designed to come into contact with patient blood and are single use, non-toxic, non-pyrogenic, supplied sterile in individual packaging. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product. Not available in all geographies. Consult your labeling.
Legal Manufacturer:
Sorin group italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) Italy