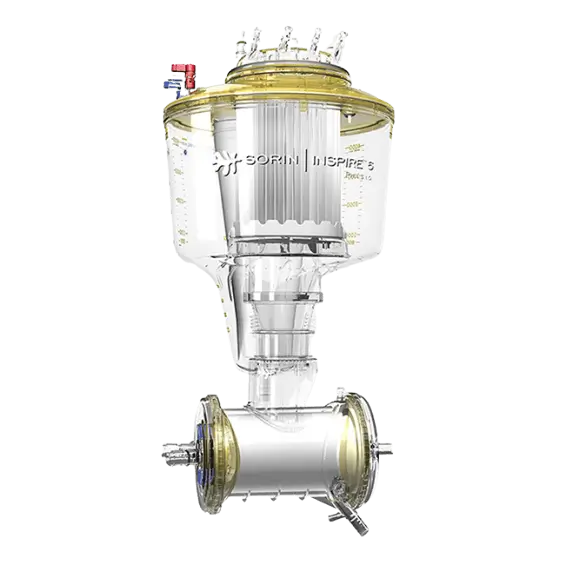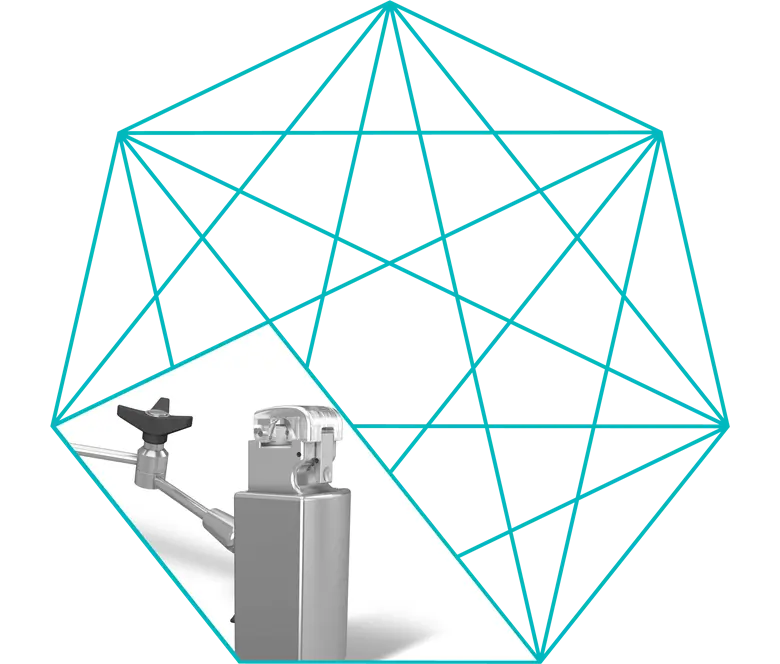
Venous Clamp
The venous clamp can apply an occlusion of perfusion tubing that varies between fully unoccluded to fully occluded.
When the pump is stopped manually, the venous clamp helps achieve controlled regulation of the venous return flow. The clamp closes automatically in case of a stopped arterial flow.
The user can also control the venous flow manually. When the arterial pump starts up, the venous clamp opens to the most recently specified set value.
An override of the stop link function is possible at any time by simply turning the control knob of the venous clamp.
The set value can be preset when the occluder is closed and the stop link function is activated.

Safety Information
1. INDICATIONS FOR USE / INTENDED PURPOSE
EU: The Stöckert S5 System /Sorin Centrifugal Pump System (CP5) /Electrical Venous Occluder (EVO) /Electrical Remote-Controlled Tubing Clamp are intended to perform, control, monitor and support extracorporeal blood circulation replacing the mechanical pumping function of the heart, monitoring and regulating physiologic parameters during procedures requiring extracorporeal circulation.
US: The Stöckert S5 System is intended to be used during cardiopulmonary bypass for procedures lasting six (6) hours or less. Canada: In accordance with the applicable regulations, the Stöckert S5 System is used to perform, control and monitor extracorporeal blood circulation during an operation.
2. CONTRAINDICATIONS
There are no known contraindications for the Stöckert S5 System.
3. WARNINGS
The device must be used in accordance with the instructions for use. For a complete listing of warnings, please refer to the Instructions for Use which accompany each product
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and thorough understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
LivaNova Deutschland
Lindberghstrasse 25
D-80939 Munich, Germany
Distributed in the USA by:
LivaNova USA
14401 W 65th Way
Arvada, CO 80004