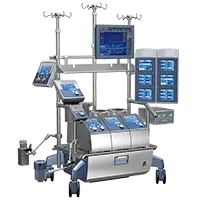
Inspire™ Family of Adult Oxygenators
Personalized perfusion and proven performance

Complete Family of Adult Oxygenators
Inspire™ is a complete family of adult oxygenators designed from years of research and laboratory experience, input from clinical experts from around the world and the application of advanced manufacturing technologies that adhere to the highest quality standards.
Prior to the introduction of Inspire, small adult devices were considered the benchmark for optimized perfusion, but were limited in flow to 5 l/min.
Inspire 6 optimized adult oxygenator systems to extend the benefits of small adult devices to a wider patient population.

Powerful, Consistent Performance
The Inspire family allows clinicians to safely run perfusion procedures while ensuring powerful, consistent performance.
- In Inspire, the long path oxygenator membrane with remarkably efficient longitudinal flow design allows high levels of gas exchange at all rated flows.
- Its polyurethane heat exchanger is capable of highly efficient heat transfer.

Low Hemodilution2
- Low dynamic operating volume (DOV).
- Inspire devices are characterized by the lowest minimum operating level in the reservoir (150 ml), outstanding low venous filter dynamic hold-up volume and low prime oxygenator modules.

Effective Gaseous Micro Emboli (GME) Control
Literature studies report correlation between embolic load and neurological deficit.3,4
- The Inspire reservoir design and oxygenator geometry contribute to GME reduction.
- The use of Inspire’s integrated arterial filter further helps improve GME removal capacity.

Designed with Reduced Blood Contact Surface Area
Inspire is designed with a reduced blood contact surface area, helping minimize blood reaction to foreign surfaces. In addition:
- LivaNova has designed a uniform biocompatible surface treatment that features phosphorylcholine (PC).4
- Inspire adult oxygenators are manufactured with NO-DEHP material to further improve biocompatibility.
- The unique design of the Dual Reservoir ensures activated suction blood sequestration.5,6

Fully modular system
The Most Complete Range of Flow Sizes for Adult Oxygenation
Inspire is a fully modular oxygenator system. The 21 different integrated models can be created by flexibly combining oxygenator modules and venous reservoirs.
Inspire HVR and HVR Dual feature one of the lowest minimum operating levels on the market, which contributes to a reduction in patient blood hemodilution.1

Safe, Easy and Flexible for All Adult Patient Sizes
The Inspire family is flexible and versatile.
- The compact oxygenator system requires minimal storage space.
- The simple bracket design allows easy and fast mounting of the oxygenator system. The bracket is available with a regular screw clamp and fast lock mechanism.
- Port orientation and system priming have been optimized to offer easy set-up and operational flexibility.
Safety Information
Summary of Safety & Performance Information for INSPIRE
1. INDICATIONS FOR USE / INTENDED PURPOSE
Inspire Oxygenator (Models: Inspire 6, Inspire 7, Inspire 8)
The devices are indicated for adult and small adult patients undergoing surgical procedures requiring cardiopulmonary bypass.
The devices are extracorporeal circulation devices used to replace the patient respiratory function during cardiopulmonary bypass procedures. They are used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the blood, and to cool or warm the blood during cardiopulmonary bypass procedures.
The devices must be to be used up to 6 hours or less.
Inspire Oxygenator with integrated arterial filter (Models: Inspire 6F, Inspire 7F, Inspire 8F)
The devices are indicated for adult and small adult patients undergoing surgical procedures requiring cardiopulmonary bypass.
The devices are extracorporeal circulation devices used to replace the patient respiratory function during cardiopulmonary bypass procedures. They are used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the blood, and to cool or warm the blood during cardiopulmonary bypass procedures. The integrated arterial filter provides filtration against air and solid emboli.
The devices must be to be used up to 6 hours or less.
Inspire Cardiotomy/venous reservoir (Models HVR, and HVR dual)
The devices are indicated for adult and small adult patients undergoing surgical procedures requiring cardiopulmonary bypass.
The devices are extracorporeal circulation devices allowing oxygenators to exploit gas exchange for the replacement of patient respiratory function during cardiopulmonary bypass procedures., providing dynamic patient’s venous blood collection. They also defoam and filter venous blood and suction blood through a filtering system made of antifoam agent and filter screen. Can be used post-operatively for chest drainage.
The devices must be to be used up to 6 hours or less
2. CONTRAINDICATIONS
No contraindications are known if the device is used for the purpose described and in accordance with the stated operating conditions. Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use provided in the Instructions for Use. . For a complete listing of warnings please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
5. ADVERSE EVENTS
The following table summarizes harms potentially arising during the use of the medical device, including those related to the intrinsic risks of extracorporeal circulation:. Systemic Inflammatory Response Syndrome (SIRS), Hypoperfusion / Hypoxia, Embolism / Hypovolemia / Sepsis / Infection / Fever / Shock/ Allergic reactions / Cyto-toxic reactions / Genetic mutation / Cancer / Haemolysis / Organ damage / Acid base imbalance (only for oxygenator) / Cross contamination / User contamination / Environment contamination / Thrombosis / Impaired hemostasis / Blood activation / Bleeding / User skin tears and Pneumothorax (only for cardiotomy/venous reservoir).
6. PERFORMANCE INFORMATION
The devices are designed to come into contact with patient blood and are single use, non-toxic, non-pyrogenic, supplied sterile in individual packaging. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use. The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website ( www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
Sorin group italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) Italy
Important Safety Information for Hardshell Venous Reservoirs
1. INDICATIONS FOR USE / INTENDED PURPOSE
The devices are indicated for adult and small adult patients undergoing surgical procedures requiring cardiopulmonary bypass.
The devices are extracorporeal circulation devices allowing oxygenators to exploit gas exchange for the replacement of patient respiratory function during cardiopulmonary bypass procedures., providing dynamic patient’s venous blood collection. They also defoam and filter venous blood and suction blood through a filtering system made of antifoam agent and filter screen. Can be used post-operatively for chest drainage.
The devices must be to be used up to 6 hours or less.
2. CONTRAINDICATIONS
No contraindications are known if the device is used for the purpose described and in accordance with the stated operating conditions. Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use. For a complete listing of warnings, please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
5. PERFORMANCE INFORMATION
The device is single use, non-toxic, non-pyrogenic, supplied STERILE and individually packaged. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use. The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website (www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) ITALY
Important Safety Information for Soft Venous Reservoirs
1. INDICATIONS FOR USE / INTENDED PURPOSE
The BMR1900 Closed Venous Reservoir Bags is intended to be used in adult surgical procedures requiring cardiopulmonary bypass procedures. It collects and handles venous blood, and suction blood from cardiotomy reservoir.
The BMR1900 is intended to be used for 6 hours or less.
The Inspire SVR 1200 is indicated for adult and small adult patients undergoing surgical procedures requiring
cardiopulmonary bypass.
The Inspire SVR 1200 is an extracorporeal circulation device allowing oxygenators to exploit gas exchange for the replacement of patient respiratory function during cardiopulmonary bypass procedures, providing dynamic patient’s venous blood collection.
The devices collect venous blood and suction blood from the cardiotomy reservoir.
The Inspire SVR 1200 must be used up to 6 hours or less.
2. CONTRAINDICATIONS
No contraindications are known if the device is used for the purpose described and in accordance with the stated operating conditions. Do not use the device for any purpose other than indicated.
3. WARNINGS
The device must be used in accordance with the instructions for use provided in the Instructions for Use. For a complete listing of warnings please refer to the Instructions for Use which accompany each product.
4. PRECAUTIONS
Federal law (U.S.A.) restricts this device to sale by or on the order of a physician. For a complete listing of precautions/cautions please refer to the Instructions for Use which accompany each product.
5. ADVERSE EVENTS
The following table summarizes harms potentially arising during the use of the medical device, including those related to the intrinsic risks of extracorporeal circulation: Hypoxia / Hypercarbia / Hypothermia / Hypoperfusion / Hypovolemia / Embolism / Sepsis / Fever / Systemic Inflammatory Response Syndrome (SIRS) / Allergic reactions / Hypersensitivity reactions / Irritation / Cytotoxic reactions / Genetic mutation / Cancer / Reprotoxic effects / Hemolysis / Impaired hemostasis / Infection (user or other persons) / Skin tears (user or other persons) / Bruise or Contusion (user) / Environmental contamination.
6. PERFORMANCE INFORMATION
The device is single use, non-toxic, non-pyrogenic, supplied STERILE and individually packaged. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website ( www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) ITALY
References
1. Habib et all- Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: Should current practice be changed- The Journal of Thoracic and Cardiovascular Surgery - June 2003
2. Ranucci M et al., Effects of priming volume reduction on allogeneic red blood cell transfusions and renal outcome after heart surgery. Perfusion 3/2015;Volume: 30: 2:120-126
3. Stehouwer MC et al., Effect of Oxygenator Size on Air Removal Characteristics: A Clinical Evaluation.ASAIO J. 2016 Jul-Aug
4. De Somer F et al. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg 2000;18:602–6
5. Shann KG et al., An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg 2006;132:283–90
6. Albes JM, et al. Physiological coagulation can be maintained in extracorporeal circulation by means of shed blood separation and coating. J Thorac Cardiovasc Surg 2003;126:1504–12
Technical claims supported by LivaNova data on file