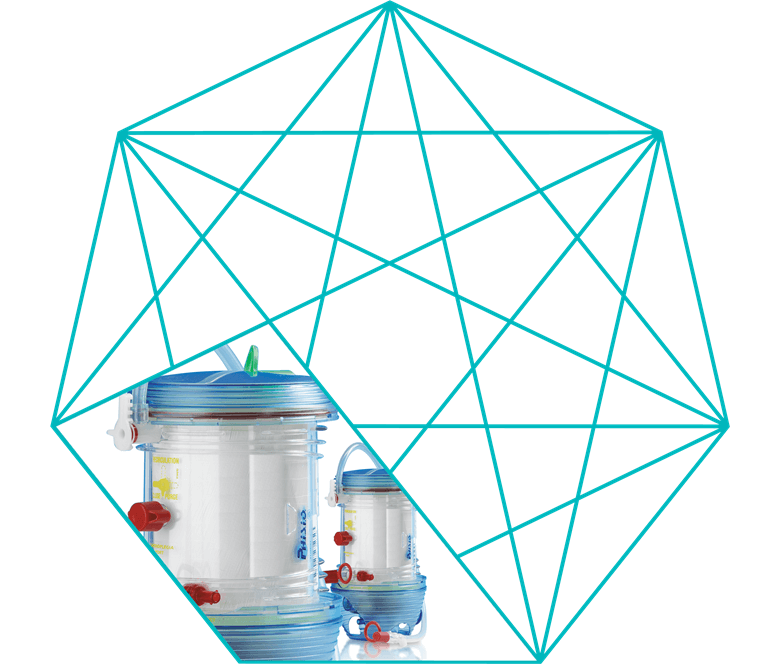
EOS ECMO
Plasma tight hollow fiber oxygenator
- EOS ECMO is by design a low priming volume oxygenator with a low membrane surface area, treated with P.H.I.S.I.O surface treatment.
- The EOS ECMO integrated heat exchanger maintains or restores normothermic blood temperature.
- The EOS ECMO purge line has an open pathway that allows continuous purging.
- LivaNova has designed a uniform biocompatible surface treatment that features phosphorylcholine (PC).

| Technical Features | |
|---|---|
| Recommended maximum blood flow (ml/min) | 5000 |
| Static priming volume (oxy module+HE) (ml) | 150 |
| Membrane surface area (sqm) | 1.2 |
| Membrane type | Hollow Fiber |
| Membrane material | Polymethylpentene |
| Heat exchanger surface area (sqm) | 0.14 |
| Heat exchanger material | Stainless Steel |
| Connections: - Venous inlet |
3/8” |
| - Arterial outlet | 3/8” |
| - Recirculation/Purge Line | 1/4” |
Safety Information
Summary of Safety & Performance Information for EOS ECMO
The devices are extracorporeal circulation devices used to partially or fully support the patient respiratory function during extended respiratory support applications and to maintain or restore normothermic blood temperature. They are used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the blood, and to warm the patient blood.
The devices must be used up to 5 days or less.
The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website ( www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) Italy
- INDICATIONS FOR USE / INTENDED PURPOSE
The devices are extracorporeal circulation devices used to partially or fully support the patient respiratory function during extended respiratory support applications and to maintain or restore normothermic blood temperature. They are used in an extracorporeal perfusion circuit to oxygenate and remove carbon dioxide from the blood, and to warm the patient blood.
The devices must be used up to 5 days or less.
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE EVENTS
6. PERFORMANCE INFORMATION
The devices are designed to come into contact with patient blood and are single use, non-toxic, non-pyrogenic, supplied sterile in individual packaging. Sterilised by ethylene oxide. The level of ethylene oxide residuals in the device is within the limits established by national regulations in the country of use.The devices should be used by qualified and skilled personnel, able to follow the indications and instructions for use contained in the information provided by the manufacturer. Please contact us through our website ( www.sorinmanuals.com) to receive instructions for use containing full prescribing information including indications, contraindications, warnings, precautions and adverse events.
The information contained in this summary represents partial excerpts taken from the product labeling. The information is not intended to serve as a substitute for a complete and through understanding of the device nor does this information represent full disclosure of all pertinent information concerning the use of this product.
Legal Manufacturer:
Sorin Group Italia S.r.l.
Via Statale 12 Nord, 86
41037 Mirandola (MO) Italy
References
1. De Somer F, Francois K, van Oeveren W, et al. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg 2000;18:602–6.
2. De Somer F, Van Belleghem Y, Caes F, Francois K, Van Overbeke H, Arnout J et al. Tissue factor as the main activator of the coagulation system during cardiopulmonary bypass. J Thorac Cardiovasc Surg 2002;123:951–8. 3. N Sohn, J Marcoux,T Mycyk, J Krahn and QH Meng1 The impact of different biocompatible coated cardiopulmonary bypass circuits on inflammatory response and oxidative stress.
