Specialty Cannulae
Available only from LivaNova Cardiopulmonary
Enhance your cannulation strategies with a diverse range of specialty options, like the ProtekDuo™ RA-PA cannula and the TandemHeart™ transseptal cannula.
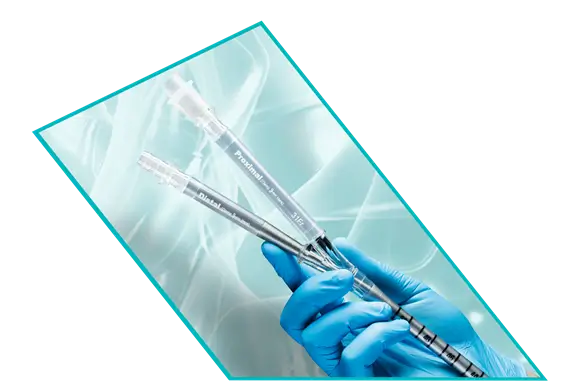
ProtekDuo™ Dual-Lumen Cannula
The Original RA-PA Bypass Cannula
The ProtekDuo cannula has a unique cannula-within-a-cannula design allows for percutaneous, single venous access at the right internal jugular vein, which provides a stable position and more patient freedom. The proximal inflow lumen is positioned in the right atrium and the distal outflow lumen is positioned in the main pulmonary artery for RA-PA bypass. The ProtekDuo cannula can be paired with your pump of choice for MCS and your pump and oxygenator of choice for ECLS.

Technical Features
|
Sizes |
31 Fr Cannula: Proximal Lumen 31 Fr x 28 cm Distal Lumen 18.5 Fr x 51 cm 29 Fr Cannula: Proximal Lumen 29 Fr x 28 cm Distal Lumen 16 Fr x 46 cm 31 Fr Rapid Deployment Cannula: Proximal Lumen 31 Fr x 28 cm Distal Lumen 18.5 Fr x 26 cm |
|---|---|
| Packaged with | One dual lumen veno-venous cannula, one introducer, and dilators. |
| Usage | Single Use Only |
| Sterilization | Ethylene Oxide Gas |
| Latex | Not manufactured with rubber latex |
| Insertion | Sold separately, the insertion kit includes one flow-directed PA catheter and one COOK® .035 Lunderquist® guidewire to enable smooth insertion of the ProtekDuo. |
TandemHeart™ Transseptal Cannula
A unique transseptal cannula that bypasses the left ventricle
The TandemHeart transseptal cannula is placed via the femoral vein. It pulls oxygenated blood from the left atrium, bypassing the left ventricle. Blood is then returned via a standard arterial cannula.

Technical Features
| Sizes |
21 Fr x 62 cm 21 Fr x 72 cm |
|---|---|
| Packaged with | One 21 Fr wire reinforced Transseptal cannula, one 14 Fr introducer, and one 14/21 Fr two stage dilator |
| Usage | Single Use Only |
| Sterilization | Ethylene Oxide Gas |
| Latex | Not manufactured with rubber latex |
ProtekSolo™ Arterial Cannula
Two sizes of cannulae, featuring a rubber stopper to reduce risk of over insertion

Technical Features
| Size |
15 Fr x 17 cm 17 Fr x 17 cm |
|---|---|
| Packaged with | One 15 Fr or 17 Fr wire reinforced arterial cannula and one 10 Fr or 12 Fr introducer |
| Usage | Single Use Only |
| Sterilization | Ethylene Oxide Gas |
| Latex | Not manufactured with rubber latex |
ProtekSolo™ Venous Cannula
A wire-reinforced venous drainage cannula

Technical Features
| Sizes |
24 Fr x 60 cm |
|---|---|
| Packaged with | One 24 Fr wire-reinforced venous drainage cannula, one 21 Fr x83 cm introducer, and dilators. |
| Usage | Single Use Only |
| Sterilization | Ethylene Oxide Gas |
| Latex | Not manufactured with rubber latex |
Picture it
How ProtekDuo Works
ProtekDuo Cannulation Animation
ProtekDuo Insertion
Important Safety Information
ProtekDuo™ Cannula Set
Brief Summary of Safety Information for the ProtekDuo™ Cannula Set
1. INDICATIONS FOR USE/INTENDED USE
ProtekDuo Veno-Venous Cannula Set is intended for use as a single cannula for both venous drainage and reinfusion of blood via an internal jugular vein during extracorporeal life support procedures.
2. CONTRAINDICATIONS
Alone, the cannula and introducer are not medical treatment devices. There are no known contraindications for the use of the cannula, other than those generally contraindicated for cardiopulmonary bypass. The cannula Introducer is only to be used with the appropriately sized ProtekDuo Veno-Venous Cannula. These devices are not intended for use except as indicated.
3. WARNINGS & PRECAUTIONS
Important: Physicians should inform patients about all potential risks and adverse events discussed in the Directions for Use. This document is not intended to serve as a substitute for the complete labeling.
The ProtekDuo Cannulae has not been qualified through in vitro, in vivo, or clinical studies for long term use (i.e., longer than 30 days) as a bridge to transplant, for pending recovery of the natural heart, or extracorporeal membrane oxygenation (ECMO).
The use of this device for periods longer than 30 days increases the risk of pump failure, reduced pumping capability, excessive blood trauma, degradation and/or corrosion of the blood contacting materials (with the possibility of the particles passing through the CPB circuit to the patient), leaks, and increased potential for gaseous emboli entering the arterial line.
Only medical personnel proficient in venous cannulation techniques and extracorporeal life support should use this device. Product is to be used in a controlled clinical environment by trained medical personnel.
Follow standard percutaneous or surgical techniques to place the device. Standard medical practice for cannula insertion site care should be followed to reduce incidence of infection.
Medical personnel responsible for patient care should minimize the length of time that System components remain inserted or attached to the blood circuit during which there is no blood flow. Examples include start-up and shut-down procedures.
4. ADVERSE EVENTS
Potential adverse events that may be associated with venous cannulation include: injury to or perforation of the myocardial wall with or without cardiac tamponade; thrombus formation; particulate or air embolism; myocardial infarction; pulmonary embolism; cardiac arrhythmias such as atrial fibrillation, heart block, sinus bradycardia, and ventricular tachycardia or fibrillation; congestive heart failure or pulmonary edema; atrial/ventricular septal defect, transient or persistent, with or without hemodynamic compromise; vascular injury with or without the need for surgical intervention; blood loss requiring fluid replacement or transfusion of blood products; infection; allergy or anaphylactic reaction to contrast media or device components; respiratory arrest; renal failure; death; failure to traverse the vascular system.
LivaNova USA | Manufactured by CardiacAssist, Inc | 620 Alpha Drive - Pittsburgh, PA 15238, USA | 866-332-1375
TandemHeart™ Transseptal Cannula Set
Brief Summary of Safety Information for the TandemHeart™ Transseptal Cannula Set
1. INDICATIONS FOR USE/INTENDED USE
The Transseptal Cannula Set is intended for transseptal catheterization of the left atrium via the femoral vein for the purpose of providing a means for temporary (six hours or less) left ventricular bypass when connected to the TandemHeart extracorporeal blood pump which returns blood to the patient via the femoral artery or other appropriate site.
2. CONTRAINDICATIONS
The Transseptal Cannula Set should not be used when any anatomical, medical, or physiological impairment may contraindicate the use of a femoral access procedure or transseptal access to the left atrium.
3. WARNINGS & PRECAUTIONS
Important: Physicians should inform patients about all potential risks and adverse events discussed in the Directions for Use. This document is not intended to serve as a substitute for the complete labeling.
The Transseptal Cannula is not intended to be extended into the left ventricle.
Product is to be used in a controlled clinical environment by trained medical personnel.
Medical personnel responsible for patient care should minimize the length of time that System components remain inserted or attached to the blood circuit during which there is no blood flow. Examples include start-up and shut-down procedures.
4. ADVERSE EVENTS
Potential adverse effects that may be associated with transseptal cannulation include the following: injury to or perforation of the myocardial wall with or without cardiac tamponade; thrombus formation; particulate or air embolism; myocardial infarction; cardiac arrhythmias such as atrial fibrillation, heart block, sinus bradycardia, and ventricular tachycardia or fibrillation; congestive heart failure or pulmonary edema; atrial septal defect, transient or persistent, with or without hemodynamic compromise; vascular injury with or without the need for surgical intervention; blood loss requiring fluid replacement or transfusion of blood products; infection; allergy or anaphylactic reaction to contrast media or device components.; loss of limb; respiratory arrest; renal failure; death; failure to traverse the vascular system.
LivaNova USA | Manufactured by CardiacAssist, Inc | 620 Alpha Drive - Pittsburgh, PA 15238, USA | 866-332-1375
ProtekSolo™ 15 and 17 Fr Arterial Cannulae
Brief Summary of Safety Information for the ProtekSolo™ 15 and 17
Fr Arterial Cannulae ProtekSolo™ 15 and 17 Fr Arterial Cannulae: The Femoral Arterial Cannula and Introducer are intended to cannulate vessels, perfuse vessels or organs, and/or connect with accessory extracorporeal circulatory support equipment for a duration of six hours or less. The cannula introducer is intended to facilitate proper insertion and placement of the cannula within the vessel for extracorporeal support. These devices are to be used by a trained physician only.
Contraindications for Use: Alone, the cannula and introducer are not medical treatment devices. There are no known contraindications for the use of the cannula, other than those generally contraindicated for cardiopulmonary bypass. The cannula introducer is only to be used with the appropriately sized TandemHeart Cannula. These devices are not intended for use, except as indicated above.
LivaNova USA | Manufactured by CardiacAssist, Inc | 620 Alpha Drive - Pittsburgh, PA 15238, USA | 866-332-1375
ProtekSolo™ Venous Cannula
Brief Summary of Safety Information for the ProtekSolo™ Venous Cannula
ProtekSolo™ Venous Cannula: The Venous Cannula and Obturator is intended to cannulate vessels, perfuse vessels or organs and/or connect with accessory extracorporeal circulatory support equipment. The cannula obturator is intended to facilitate proper insertion and placement of the cannula within the vessel for extracorporeal circulatory support. These devices are to be used by a trained physician only.
Contraindications for Use: Alone, the cannula and introducer are not medical treatment devices. There are no known contraindications for the use of the cannula, other than those generally contraindicated for cardiopulmonary bypass. The introducer is only to be used with the appropriately sized Venous Cannula. These devices are not intended for use except as indicated above.
LivaNova USA | Manufactured by CardiacAssist, Inc | 620 Alpha Drive - Pittsburgh, PA 15238, USA | 866-332-1375

